Green Mountain Antibodies on Antibody Characterization
Green Mountain Antibodies is the leading antibody producer and validator for over two decades now. We have grown a team of experts in departments ranging from biology, chemistry to application development. We aim to maintain our legacy of being the top antibody producers in the United States and Earth for perpetuity. We provide several services related to antibodies in our company, namely the complete characterization of antibodies. We are fully equipped to carry out this thorough procedure using multiple methods for routine quality assurance and as a third-party reference. The information that is described in this article will inform you on what is required for antibody isotyping, as well as the characterization methods that are carried out within our lab.
What Is Antibody Characterization?
Antibody characterization is very essential in carrying out research and development for the majority of biopharmaceutical products. Even the latest in technological advances utilized in the production of therapeutics have heterogeneities in the finished good (commercially available product) because several variables could occur during each of the several stages of production. Complete and concise characterization with a clear conclusion are more important than ever for the reproducible and safe production of therapeutic proteins because of the great potential for heterogeneities.
Protein and antibody characterization is also important for the development and creation of successful assays used for discovery. In order to make these assays reproducible it is important to characterize the various properties of antibodies, such as the chemical properties, purity and impurity of the antibodies, and exact quantities.
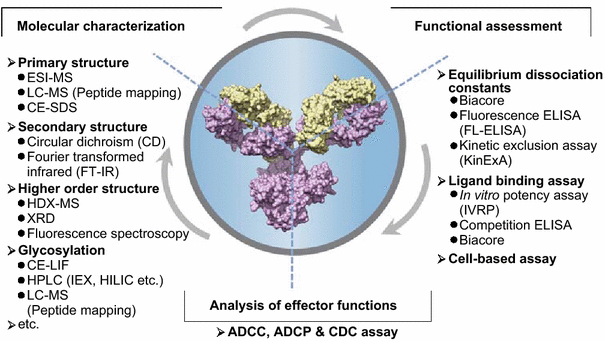
Monoclonal antibody (mAb) characterization and analysis can be carried out using different methods. Our labs at Green Mountain Antibodies carry out the process in 5 unique antibody characterization techniques, for different purposes and applications.
Labeling Antibodies Using Fluorescent And Biotin Markings:
One accurate technique we employ for the characterization of antibodies at GMAb exploits Antibody Labeling with Biotin and Fluorescent Labels (ALBFL). Labeled antibodies are commonly required in assays, and this is one of many customizations that GMAb offers to its clients. Using this technique antibodies can be analyzed and labeled based on the signals that they exhibit. Some common applications of this technique are to make antibodies to be used in optically measured assays (OMA). It also helps reduce background and increases assay sensitivity which is particularly useful for identifying the ideal hybridomas in situations where the antigen is a limiting factor.
Use Of SEC Or Light Scattering Techniques For Aggregation Analysis:
Light scattering and size exclusion chromatography (SEC) are two very complimentary ways to analyze and categorize antibodies. These techniques can be applied to measure the moles, the ratio of antibodies to antigen, and how they are going to work stoichiometrically in a particular assay. Using dynamic light scattering and diffusion allows for the size of the antibody to be measured accurately. While static light scattering alone can be used to find the moles and the radius of the antibody. SEC is used to separate the antibodies based on their size based on exceptionally minute differences. The methods are applied to detect antibody concentration with extreme precision antibody and even indicate the heterogeneousness of the antibody.
Flow Cytometry:
We listened to our customers and responded to the need for innovation and additional screening capabilities for on-cell binding. Part of our response was to bring in an Attune flow cytometer at the end of 2020. Since then, GMAb has refined and optimized the service to offer the new technology throughout the hybridoma development process. This is effort is to assure our customers find the best antibody for their end application, using the fastest and most affordable method.
Flow cytometry is a necessary application for therapeutic customers looking to develop a monoclonal antibody for the following types of projects:
- CAR-T specific detection
- Anti-ID NAb assays for cell surface markers where soluble antigen is not available
- Confirm cell binding in real time for therapeutic targets
- Measure mAb internalization
Octet Analysis:
The Octet Analysis method used at GMAb incorporates Bio-Layer Interferometry (BLI) to measure the bi-molecular interactions between antibody and antigen. Using this method we offer Koff ranking along with matched pair data for the selected antibodies.
Antibody Sequencing:
Even though all antibodies have the same basic protein structure, their sequence variability in the regions of antigen – paratope region vary significantly. Surprisingly, the sequence variability of two antibodies derived from different hybridoma cells to the same antigen also differ considerably. At GMAb we can characterize antibodies by labeling these different sequence variations and identifying the ones worth developing for downstream applications.
Fragmentation:
Preparation of Fab and F(ab’)2: Characterization using fragmentation technique is also a method of antibody characterization used at the GMAb. This technique is applied for making it easier for the antibodies to penetrate the tissue to detect the permeability and specificity of the antigen binding region.
Contact us: Contact Green Mountain Antibodies for a full range of services for creating new monoclonal antibodies at 802-865-6230.






