The Practicalities of Monoclonal Antibody Development
It has been 3 decades since the very first monoclonal antibody was approved as a therapeutic for intravenous administration to combat cancer and since that point, a lot has evolved in the field of monoclonal antibody production for therapeutic applications. These monoclonal antibodies are used now to treat various diseases such as cancer, autoimmune, and other infectious diseases. However, when we still hear about them, we often get confused between monoclonal antibody development and antibody production. Hence Green Mountain Antibodies, one of the leading companies in the industry with decades of experience in creating monoclonal antibodies, is here to give you clarity about these 2 procedures.
- Q) Explain Development vs Production of Antibodies:
Antibodies are first developed by host species and with the help of procedures like hybridization, splenocytes can be immortalized to produce monoclonal antibodies in conjunction with bioreactors. After that, the fully developed antibodies are produced further by growing up batches of hybridoma cells as needed. It means, one procedure is just an extension of another one as development leads to production which can lead to further development in a never-ending cycle to control quality and cost.
- Q) What is the meaning of Antibody development?
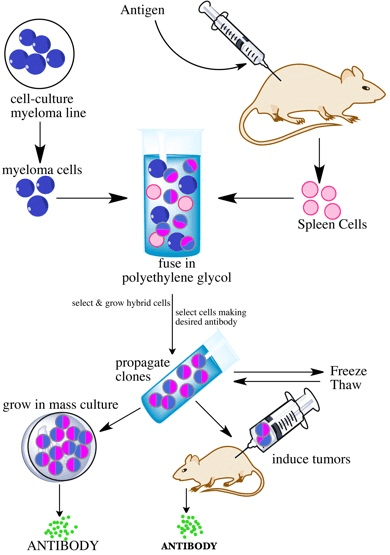
Antibody development begins with first injecting an antigen into a host animal. It targets the immune system and develops numerous combinations of antibodies. At that point, antibodies are validated for specificity, affinity, and stability. The selection process involves identifying the cell line responsible for generating the highest performing antibodies. Once the selection process is done, the production procedures begin along with preserving the genetic information underlying the formation of the desired antibody.
- Q) What is meant by the Production of Antibodies?
In the production process, technicians are attempting to get the highest yields of antibodies possible without forming aggregates, precipitates, or other variants that could affect performance. The production procedure changes depending upon the type of antibody:
- Polyclonal Antibody: These antibodies are harvested in an antiserum from a host animal. It means the whole serum from which the antibody is derived is bled from an animal and then purified. However, for larger quantities of polyclonal antibodies, a larger animal must be used such as a goat or sheep.
- Monoclonal Antibody: Unlike polyclonal antibodies which are produced in live animals, monoclonal antibodies are developed using in vitro or in vivo cell culture techniques. It typically involves taking immortalized cell lines that produce the antibodies of interest and growing them at scale using specialized equipment including bioreactors.
- Q) What is meant by the Development of Monoclonal Antibody?
- In Vitro Production: In this process, antibodies are produced by growing already developed hybridomas in cultural facilitation.
- In Vivo Production: This is a much complex process. It involves injecting the developed hybridomas into a mouse peritoneum and provide it with additional nutrients for faster proliferation. This technique is obviously a challenge to do at scale for commercial applications.
- Q) Why do Developed Antibodies need further production?
The primary goal of antibody development is to isolate the best antibody from the immune system using techniques like phage display or plasma cell cloning. And the aim of further production is to get the desired type of antibodies for specified applications in large quantities.
- Q) Are developed Monoclonal Antibodies safe?
These are considered one of the safest types of bio-therapeutics. In fact, every year hundreds of thousands of new antibodies are approved by FDA for clinical purposes. With that being said, side effects of these antibodies are possible to occur, but it only happens in an extremely low number of patients.
- Q) How long does it take to develop Monoclonal Antibodies?
The time required in their development process depends upon how immunogenic the antigen is as well as the type of antibody that is being developed. But the most complex and time-consuming projects are usually the ones that are being manufactured for commercial purposes, such as bio- therapeutic and diagnostic antibodies for medical devices.
Since the complex nature of monoclonal antibodies, you must look for a reliable company with the best record in developing and producing monoclonal antibodies. The difference between working with experienced scientists vs inexperienced scientists will cost years in getting to a fully viable commercial monoclonal antibody. That is why monoclonal antibody development is no trivial matter.
Contact Green Mountain Antibodies for a full range of services for creating new monoclonal antibodies or producing antibodies: 802-865-6230






