Producing a Complex Molecule
Antibody production of recombinant antibodies is a different process than creating new monoclonal antibodies. Producing monoclonal antibodies from existing hybridoma cell lines is a rigorous process from start to finish with the possibility of cell lines crashing and losing an entire batch. Antibody sequencing is a guarantee that the information for generating highly specific antibodies is preserved.
Green Mountain Antibodies is a thoroughly experienced preeminent antibody production company that offers a range of services in either developing monoclonal antibodies de novo or generating them from existing hybridomas. Antibody sequencing is a service we offer to ensure the quality of your antibodies is preserved for small batch to large-scale production.
Our team can bring value to any part of the entire process, and we can come in at any point to bring a project to completion. We have produced an extensive line of readily available purified antibodies that are being used by the top pharmaceutical companies because of the quality. We are top-tier, antibody developers that bring our expertise into every custom project. Of course, there’s antibody development, and then there is ensuring the success of the antibodies in the required application, and for that, we utilize a full assortment of techniques. Antibody sequencing is a data-intensive technique that enables long-term success.
A Simplified Workflow for Monoclonal Antibody Sequencing
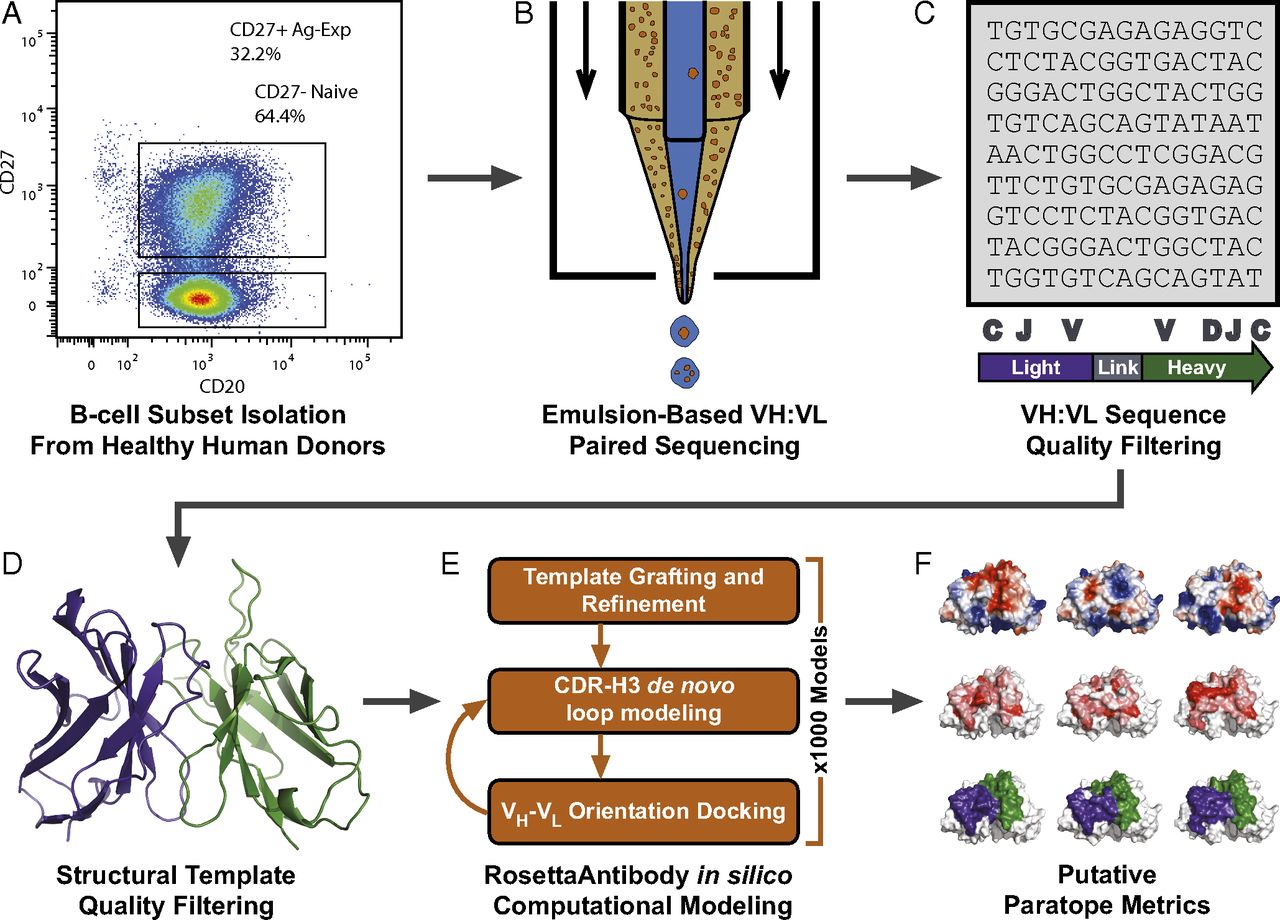
Antibody sequencing, a technique that is part of antibody characterization, involves the derivation of amino acid sequences from an antibody with mass spectrometry. The diversity of variable antibody regions usually makes the sequencing a little more tedious and challenging since no two variable regions are the same (even from a monoclonal population).
Ultimately, a prime reverse transcription is done alongside a respective primer with a constant region. Afterward, a custom sequence is created at the 5′ end of the antibody cDNA using a template switch oligonucleotide. The template switching allows for a chance to escape the issue of low sequence homology or the need for degenerate primers. In subsequent PCR amplification of antibody cDNA molecules, only two primers are required: one primer specific for the template switch oligonucleotide sequence and another for a nested primer for the respective constant region.
In one scenario, chimeric mouse/human antibody expression plasmids for recombinant antibody production in mammalian cell culture expression systems (CHO cells) are designed by using the cutting-edge method of sequencing five mouse monoclonal IgG antibodies’ variable regions and combine their genetic information via advanced computer algorithms. This can also be done by sequencing mouse monoclonal antibodies which is the service we offer at GMAb.
How is the amino acid sequence determined?
When all the five recombinant antibodies have been bounded with their respective antigens with high affinity, it is a confirmation that the amino acid sequences and the method used are correct.
Our engineering and production services at Green Mountain Antibodies are leveraging software that allows for quicker and more effective results. Our expertise in developing software is a tremendous advantage that ensures the successful data analysis of the antibody sequencing output, along with other aspects of the development process leads to the effective production of antibodies.
The Green Mountain Antibodies team is comprised of dedicated professionals; trained and experienced scientists who make each project enjoyable, efficient, and worthwhile. GMAb is at the forefront of technological advances in an industry-leading the way to major scientific breakthroughs and facilitating these efforts through the manufacturing of antibodies for therapeutics, diagnostics, and research.
Our staff of expert scientists has specialized in the production of monoclonal and polyclonal antibodies. The reliable production of antibodies is one of the world’s most pressing health challenges, and we professionally passionately help provide a solution to these urgent needs.
Antibody sequencing helps to protect your antibody from loss of fidelity and enhance the full capabilities of your antibodies. It helps prevent the loss of the inherent value contained within hybridoma cells, as well. All this information is essential for you so you can take the right step in your most important projects. Contact Green Mountain Antibodies for a full range of services for the successful development of a new monoclonal and/or polyclonal antibody: 802-865-6230.






